Abstract
OBJECTIVES: Tyrosine kinase inhibitors (TKIs) are the standard of care for adult and pediatric patients with chronic myeloid leukemia in chronic phase (CML-CP). More treatment options with improved efficacy and safety profiles are needed for pediatric patients. Asciminib is a first-in-class allosteric inhibitor of ABL kinases, Specifically Targeting the ABL Myristoyl Pocket (STAMP), and does not inhibit other protein kinases. Asciminib has been approved by the FDA with subsequent approvals worldwide for patients with Philadelphia chromosome-positive (Ph+) CML-CP treated with ≥2 prior TKIs. Asciminib 40 mg twice daily (BID) has demonstrated improved major molecular response rates (25.5% vs 13.2% at Week 24, respectively) as well as a favorable safety profile compared with bosutinib 500 mg once daily in adult patients with Ph+ CML-CP in the phase III ASCEMBL study (NCT03106779). The phase Ib/II ASC4KIDS study aims to characterize the pharmacokinetics (PK) and long-term safety profile of asciminib in pediatric patients and identify a pediatric formulation dose leading to asciminib exposure comparable to 40 mg BID in adult patients.
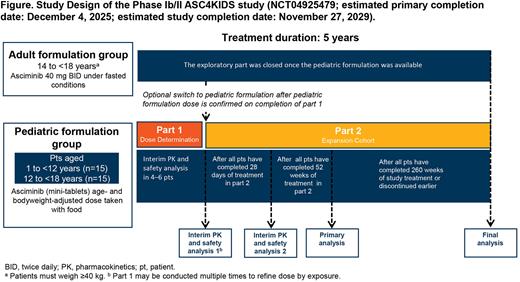
METHODS: This multicenter, open-label study (NCT04925479) includes patients aged 1 to <18 years with Ph+ CML-PC, without the T315I mutation, treated with ≥ prior TKIs. In the pediatric formulation group, at least 15 patients will be included in each of the age groups: 1 to <12 years and 12 to <18 years. The study started with an exploratory group of adolescent patients (aged 14 to <18 years and weighing ≥40 kg) treated with the adult dose and formulation (40 mg tablet BID) under fasting conditions. Once the endpoints include a primary endpoint PK, hematologic and molecular responses, safety, and acceptability / palatability of the pediatric formulation.
Part 1 (dose-determining cohort): 4 to 6 patients will receive the asciminib pediatric formulation (mini-tablet 1.3 mg/kg with food) to assess the safety and whether the exposure in pediatric patients is comparable to that with the adult formulation over the first 28 days of treatment. After the interim analysis, if a dose adjustment is required, an additional 4 to 6 patients will be assessed at an adjusted dose.
Part 2 (expansion cohort): the rest of the 30 patients will receive the dose determined in Part 1. The second interim PK and safety analysis will be conducted after all patients have completed 28 days of treatment.
The PK, safety profile, and pharmacodynamics endpoints will be assessed after all patients have completed 52 weeks of treatment. The total treatment period is 5 years (Figure). A final analysis will be performed after all patients have completed treatment. Patients who discontinue treatment early will be followed up for survival until the study is completed.
There are 34 study sites in 14 countries worldwide participating in this study. Among these sites, 21 are currently recruiting in France, Germany, Greece, Hungary, Italy, Japan, Poland, Thailand, Turkey, and the US.
RESULTS: The exploratory group recruited three patients (data cutoff: July 28, 2022).
CONCLUSIONS: This study will determine a pediatric dose for asciminib and support a strategy of full extrapolation from adult data to use in the pediatric population.
This study is sponsored by Novartis.
Disclosures
Hijiya:Novartis: Research Funding; Pfizer: Research Funding; Incyte: Honoraria, Other: Data monitoring committee; Stemline Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kapoor:Novartis: Current Employment, Current equity holder in private company. Hoch:Novartis: Current Employment, Current equity holder in private company. Descamps:Novartis: Current Employment. Dasgupta:Novartis: Current Employment. Ramscar:Novartis: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal